Tofacitinib citrate bụ ọgwụ ndenye ọgwụ (aha ahia Xeljanz) nke Pfizer mepụtara maka otu klas Janus kinase (JAK) inhibitors. Ọ nwere ike họrọ igbochi JAK kinase, gbochie ụzọ JAK / STAT, wee si otú ahụ gbochie ntụgharị mgbaàmà cell na okwu mkpụrụ ndụ metụtara ya na ịgbalite, nke a na-eji agwọ ọrịa ogbu na nkwonkwo, ọrịa ogbu na nkwonkwo psoriatic, ulcerative colitis na ọrịa ndị ọzọ na-alụso ọrịa ọgụ.
Ọgwụ na-agụnye ụdị usoro onunu ogwu atọ: mbadamba nkume, mbadamba ihe ntọhapụ na ngwọta ọnụ. Mbadamba ụrọ ya bụ ndị FDA kwadoro na mbụ na 2012, na FDA kwadoro ụdị ọgwụgwọ a kwadoro na February 2016. Ọ bụ nke mbụ na-agwọ nkwonkwo rheumatoid. Yan bụ ihe mgbochi JAK nke a na-ewere ọnụ otu ugboro n'ụbọchị. N’ọnwa Disemba afọ 2019, akwadoro ihe ngosi ọhụrụ maka ọgwụ na-adịgide adịgide maka ọnya ọnya ọnya na-arụsi ọrụ ike (UC). Na mgbakwunye, nke ugbu a na-adọ 3 clinical ule maka plaque psoriasis e dechara, na ọzọ isii nkeji 3 ụlọ ọgwụ ule na-aga n'ihu, metụtara psoriatic ogbu na nkwonkwo na-arụ ọrụ, ụmụaka idiopathic ogbu na nkwonkwo, wdg Ụdị egosi. Uru nke mbadamba ụrọ na-ewepụta oge na-arụ ọrụ ogologo oge ma ọ dị mkpa ka ewere ya otu ugboro n'ụbọchị na-enyere aka njikwa na njikwa nke ọrịa ndị ọrịa.
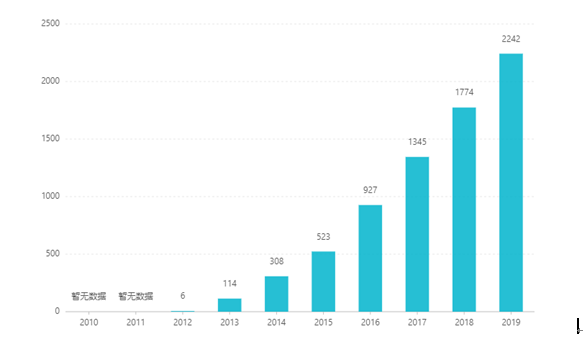
Ebe ọ bụ na ndepụta ya, ahịa ya abawanyela site n'afọ ruo n'afọ, na-eru ijeri US $ 2.242 na 2019. Na China, akwadoro ụdị doseji mbadamba maka ịzụ ahịa na March 2017, wee banye na mkpuchi mkpuchi ahụike B katalọgụ site na mkparịta ụka na 2019. Mmeri kachasị ọhụrụ. Ọnụ ahịa ya bụ 26.79 RMB. Agbanyeghị, n'ihi nnukwu ihe mgbochi teknụzụ dị elu nke nkwadebe mwepụta na-adịgide adịgide, enwetabeghị ụdị usoro onunu ogwu a na China.
JAK kinase na-ekere òkè dị mkpa na mbufụt, na ndị na-egbochi ya egosiwo na ha na-agwọ ụfọdụ ọrịa na-egbuke egbuke na autoimmune. Ruo ugbu a, 7 JAK inhibitors akwadoro n'ụwa niile, gụnyere Leo Pharma's Delgocitinib, Celgene's Fedratinib, AbbVie's upatinib, Astellas's Pefitinib, Eli Lilly's Baritinib na Novartis's Rocotinib. Agbanyeghị, naanị tofacitinib, baritinib na rocotinib ka akwadoro na China n'ime ọgwụ ndị a kpọtụrụ aha n'elu. Anyị na-atụ anya ka anabatara mbadamba ụrọ nke Tofatib Citrate nke Qilu ozugbo enwere ike ma na-erite uru karịa ndị ọrịa.
Na China, NMPA kwadoro nyocha mbụ tofacitib citrate na March 2017 maka ọgwụgwọ nke ndị okenye RA ndị ọrịa na-ezughị oke ma ọ bụ enweghị ndidi na methotrexate, n'okpuru aha ahia Shangjie. Dabere na data sitere na Meinenet, ire nke mbadamba ụrọ tofacitib citrate na ụlọ ọrụ ahụike ọha na China na 2018 bụ nde yuan 8.34, nke dị ala karịa ahịa ya zuru ụwa ọnụ. Akụkụ buru ibu nke ihe kpatara ya bụ ọnụahịa. A na-akọ na ọnụ ahịa azụmaahịa mbụ Shangjie bụ yuan 2085 (mbadamba 5mg*28), ọnụ ahịa ya kwa ọnwa bụ yuan 4170, nke na-abụghị obere ibu maka ezinụlọ nkịtị.
Otú ọ dị, ọ bara uru ime ememe na tofacitib gụnyere na 2019 "National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List" site na National Medical Insurance Administration mgbe mkparịta ụka na November 2019. A na-akọ na a ga-ebelata ụgwọ ọnwa kwa ọnwa. ruo n'okpuru 2,000 yuan mgbe a kparịtara mbelata ọnụahịa, nke ga-eme ka nnweta ọgwụ ahụ dịkwuo mma.
N'August 2018, Board Patent Reexamination Board nke State Intellectual Property Office mere a review mkpebi No. 36902 arịrịọ maka invalidation, na kwuputara emebighị isi patent nke Pfizertofatib, compound patent, na mgbakwasị nke ezughị ezu ngosi nke nkọwapụta. Agbanyeghị, patent nke ụdị kristal Pfizertofatib (ZL02823587.8, CN1325498C, ụbọchị ngwa 2002.11.25) ga-agwụ na 2022.
Ebe nchekwa data Insight na-egosi na, na mgbakwunye na nyocha mbụ ahụ, akwadoro ọgwụ ise nke Chia Tai Tianqing, Qilu, Kelun, Osimiri Yangtze, na Nanjing Chia Tai Tianqing maka ịzụ ahịa n'ime usoro mbadamba ụrọ tofacitinib. Otú ọ dị, maka ụdị mbadamba ihe na-adịgide adịgide, ọ bụ naanị nchọpụta mbụ Pfizer debere ngwa ahịa na May 26. Qilu bụ ụlọ ọrụ mbụ na-enyefe ngwa ahịa maka usoro a. Na mgbakwunye, CSPC Ouyi nọ n'ọkwa ikpe BE.
Changzhou Pharmaceutical Factory (CPF) bụ onye na-eduga na-emepụta ọgwụ API, emechara usoro na China, nke dị na Changzhou, mpaghara Jiangsu. E hiwere CPF na 1949. Anyị etinyela aka na Tofacitinib Citrate site na 2013, wee nyefee DMF ugbua. Anyị edebanyela aha n'ọtụtụ mba, ma nwee ike ịkwado gị nkwado akwụkwọ kacha mma maka Tofacitinib Citrate.
Oge nzipu: Jul-23-2021