Dabigatran Etexlate Mesylate
Nkọwa
Dabigatran etexilate mesylate (BIBR 1048MS) bụ ọgwụ na-arụsi ọrụ ike nke Dabigatran. Dabigatran etexilate mesylate nwere mmetụta anticoagulant ma jiri ya mee ihe maka prophylaxis nke venousthromboembolism na ọrịa strok n'ihi fibrillation atrial.
ndabere
Nkọwa: IC50 Uru: 4.5nM (Ki); 10nM (Mkpokọta platelet na-ebute Thrombin) [1] Dabigatran bụ ihe ntụgharị na nhọrọ, onye na-emechi thrombin kpọmkwem (DTI) na-enwe mmepe ụlọ ọgwụ dị elu dị ka prodrug na-arụ ọrụ ọnụ, dabigatran etexilate. in vitro: Dabigatran ahọrọ na ngbanwe na-egbochi thrombin mmadụ (Ki: 4.5 nM) yana thrombin-induced platelet aggregation (IC(50): 10 nM), ebe ọ na-egosi enweghị mmetụta mgbochi na ndị ọzọ na-akpali akpali platelet. Thrombin ọgbọ na platelet. Plasma dara ogbenye (PPP), nke a tụrụ ka egbochiri ikike thrombin endogenous (ETP). dabere na ntinye uche (IC(50): 0.56 microM). Dabigatran gosipụtara mmetụta anticoagulant na-adabere na ntinye uche na ụdị dị iche iche na vitro, na-eme ka oge thromboplastin na-arụ ọrụ (aPTT), oge prothrombin (PT) na ecarin clotting oge (ECT) na PPP mmadụ na ntinye nke 0.23, 0.83 na 0.18 microM, n'otu n'otu. 1]. na vivo: Dabigatran gbatịpụrụ dose aPTT dabere na nchịkwa eriri afọ na oke (0.3, 1 na 3 mg / kg) na enwe rhesus (0.15, 0.3 na 0.6 mg / kg). Ahụrụ mmetụta ọgwụ mgbochi ọgwụ na oge dabere na dabigatran etexilate a na-enye oke oke n'ọnụ (10, 20 na 50 mg / kg) ma ọ bụ enwe rhesus (1, 2.5 ma ọ bụ 5 mg / kg), yana nsonaazụ kachasị dị n'etiti 30 na 120. min mgbe nchịkwachara, n'otu n'otu [1]. Ndị ọrịa a na-emeso dabigatran etexilate nwere obere ọrịa strok ischemic (3.74 dabigatran etexilate vs 3.97 warfarin) yana obere ọbara ọgbụgba intracranial jikọtara ọnụ na ọrịa strok hemorrhagic (0.43 dabigatran etexilate vs 0.99 warfarin) kwa afọ 1020. Nnwale ụlọ ọgwụ: Nyocha nke Pharmacokinetics na Pharmacodynamics nke Oral Dabigatran Etexilate na ndị ọrịa Hemodialysis. Nkeji1
Nchekwa
| Ntụ ntụ | -20°C | Afọ 3 |
| 4°C | afọ 2 | |
| Na ihe mgbaze | -80 Celsius | Ọnwa isii |
| -20°C | 1 ọnwa |
Ọnwụnwa ụlọọgwụ
| Nọmba NCT | Onye nkwado | Ọnọdụ | Ụbọchị mmalite | Usoro |
| NCT02170792 | Boehringer Ingelheim | Ahụ ike | Febụwarị 2001 | Agba 1 |
| NCT02170974 | Boehringer Ingelheim | Ahụ ike | Julaị 2004 | Agba 1 |
| NCT02170831 | Boehringer Ingelheim | Ahụ ike | Mee 1999 | Agba 1 |
| NCT02170805 | Boehringer Ingelheim | Ahụ ike | Eprel 2001 | Agba 1 |
| NCT02170610 | Boehringer Ingelheim | Ahụ ike | Maachị 2002 | Agba 1 |
| NCT02170909 | Boehringer Ingelheim | Ahụ ike | Disemba 2004 | Agba 1 |
| NCT02171000 | Boehringer Ingelheim | Ahụ ike | Eprel 2005 | Agba 1 |
| NCT02170844 | Boehringer Ingelheim | Ahụ ike | Ọnwa Isii 2004 | Agba 1 |
| NCT02170584 | Boehringer Ingelheim | Ahụ ike | Jenụwarị 2001 | Agba 1 |
| NCT02170935 | Boehringer Ingelheim | Thromboembolism Venous | Eprel 2002 | Agba 2 |
| NCT02170636 | Boehringer Ingelheim | Ahụ ike | Jenụwarị 2002 | Agba 1 |
| NCT02170766 | Boehringer Ingelheim | Ahụ ike | Ọktoba 2000 | Agba 1 |
| NCT02171442 | Boehringer Ingelheim | Ahụ ike | Eprel 2002 | Agba 1 |
| NCT02170896 | Boehringer Ingelheim | Ahụ ike | Ọktoba 2001 | Agba 1 |
| NCT02173730 | Boehringer Ingelheim | Ahụ ike | Nọvemba 2002 | Agba 1 |
| NCT02170623 | Boehringer Ingelheim | Ahụ ike | Febụwarị 2002 | Agba 1 |
| NCT02170116 | Boehringer Ingelheim | Ahụ ike | Nọvemba 1998 | Agba 1 |
| NCT02170597 | Boehringer Ingelheim | Ahụ ike | Ọgọst 2003 | Agba 1 |
| NCT01225822 | Boehringer Ingelheim | Thromboembolism Venous | Nọvemba 2002 | Agba 2 |
| NCT02170701 | Boehringer Ingelheim | Thromboembolism Venous | Ọktoba 2000 | Agba 2 |
| NCT02170740 | Boehringer Ingelheim | Ahụ ike | Nọvemba 1999 | Agba 1 |
| NCT02170922 | Boehringer Ingelheim | Ahụ ike | Julaị 1999 | Agba 1 |
Ọdịdị chemical
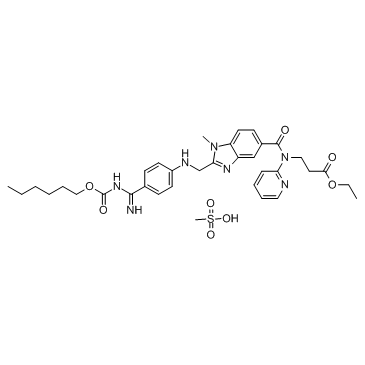





Atụmatụ18Arụmọrụ ntule na-agbanwe agbanwe nke akwadoro4, na6oru ngo na-akwado.

Usoro njikwa ogo mba ụwa dị elu etinyela ntọala siri ike maka ire ahịa.

Nlekọta dị mma na-aga n'ihu na usoro ndụ nke ngwaahịa ahụ niile iji hụ na ịdị mma na mmetụta ọgwụgwọ.

Ndị otu Regulatory Affairs ndị ọkachamara na-akwado ịdịmma chọrọ n'oge ngwa na ndebanye aha.


Ahịrị nkwakọ ngwaahịa Korea Countec


Taiwan CVC ahịrị nkwakọ ngwaahịa


Line CAM Board nke Italy

Igwe na-agbakọ Fette German

Ihe nchọpụta mbadamba ihe ngosi nke Japan

Ụlọ njikwa DCS







